In 2019, a significant change in the standards and processes around sterile medication compounding and hazardous drug (HD) handling was expected by hospital pharmacy professionals. The revision of USP <797> and the enforceability of USP <800> were slated for December 1, 2019, but for various reasons this change has been delayed. Currently, the 2008 version of <797> is still official. <800> is published, and its standards are considered to be best practices, but they are also not currently enforceable on a national level. Hospital and health-system pharmacies may find themselves in a position of uncertainty related to the proper environment, monitoring, and practices for sterile compounding and HD handling.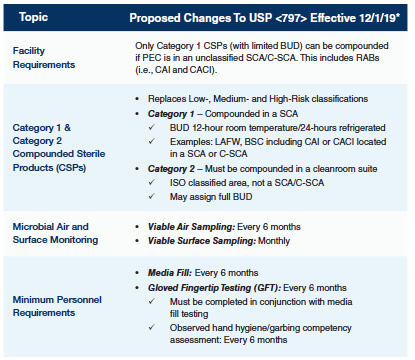 Support for Compliance
Support for Compliance
With 50 years of pharmacy expertise, Comprehensive Pharmacy Services (CPS) guides hospitals and assists them to ensure compliance today and in the future with sterile compounding and HD handling standards.
A hospital system Chief Pharmacy Officer, who previously partnered with CPS, brought CPS in to complete an initial assessment and evaluate USP and compliance across 10 pharmacies in hospitals and infusion centers across their large hospital system.
PHASE 1: Assessment
CPS assigned a sterile compounding expert to spend one day at each of the 10 sites reviewing USP and standards in the following areas:
- Physical plant and equipment
- Work practices
- Competencies and training
- Environmental monitoring
- Quality assurance activities and documentation
Because CPS sterile compounding experts are pharmacists with prior experience in hospital and health system pharmacies, they bring a holistic approach to the issues that directors of pharmacy and their staff face. They can assign a staff pharmacist or lead technician to serve as liaison with the CPS sterile compounding expert.
“Because we’ve done so many of these assessments in so many hospitals and large health systems, we’ve become very proficient and can get information very quickly. In addition, we take a non-invasive and collaborative approach to conducting assessments that minimally interrupts the work flow, while being educational to staff,” said Chris Beebe, Division Vice President, Compliance and Regulatory Services for CPS.
In this case, each site received a full report and action plan that identified areas of non‑compliance or partial compliance, along with strategies to correct those areas following the assessments.
PHASE 2: Continuous Readiness Implementation
The action plans identified multiple opportunities for improvement and standardization across the 10 sites. The Chief Pharmacy Officer recognized the need for a single point of contact to coordinate the implementation of the recommended changes and standardization across the hospitals and infusion centers. They selected the CPS expert to function as that single point of contact.
The implementation phase is an ongoing effort that encompasses three years. This is in order to ensure compliance. During the implementation phase, the CPS sterile compounding expert is on-site three days every month to:
- Track progress on the action plan at each location
- Work with corporate pharmacy leadership to implement standardization throughout the hospital system
- Interface with architects and engineers tasked with building new pharmacies and/or making changes to existing pharmacies to ensure that the construction of physical space is in compliance with USP standards
To standardize processes, the CPS expert works with system departments, including:
- Environmental services and housekeeping leadership to implement a standardized IV room cleaning program across the enterprise
- Infection prevention department to standardize the environmental monitoring program
- Regulatory affairs department to assist with survey preparedness related to USP
- Education department to standardize pharmacy competency training
The CPS sterile compounding expert also leads the system hazardous drug task force to implement strategies related to USP compliance covering assessment of risk, preparation and administration of hazardous drugs, and education of all staff involved in the process. The CPS expert is on tap for support if the system has an accreditation survey issue related to USP during the continuous readiness engagement. By participating in the CPS Continuous Readiness Program, the system also has access to the CPS Sterile Compounding Toolkit, which typically is only available to CPS managed sites.
“Our toolkit includes a standardized assessment of risk for hazardous drugs on a hospital’s formulary,” said Beebe. “It’s an important tool for hospitals and was developed to be a comprehensive yet straightforward method to assess, document, and communicate the containment strategies a hospital will employ for these drugs.”
CONCLUSION
Following the initial sterile compounding assessments and ongoing continuous readiness process, including implementation of the action plans, subsequent regulatory surveys at system sites resulted in no USP-related findings.
It is recommended that hospitals and health systems evaluate their processes and implement actions to ensure that they are as compliant as possible with USP <797> and <800> regulations. CPS brings a breadth of experience in hospitals of all types and sizes. Because we understand what surveyors are looking for, they have the expertise to set client hospitals and health systems up with a continuous readiness program that ensures their success.
For more information, contact CPS: contactus@cpspharm.com.

 All Posts
All Posts