February 2024
Pharmacy compliance expert, Chris Beebe, continues his USP Chapter <797> series with an exploration of what's clearer about the revised standards launched in November 2023.
By: Chris Beebe, Senior Vice President, Compliance and Regulatory Services
We’re still in the first few months of revised USP Chapter <797> and I’m seeing lots of progress at our hospitals. Recently, I’ve blogged about the revisions, focusing on four categories to help us keep track of the changes:
- What’s new about the revisions?
- What’s more challenging about the revisions? (With a "bonus" blog post including a few extra tips for you.)
- What's clearer about the revisions?
- What's easier about the revisions?
Previously, we covered the new and the potentially challenging changes. Now, let's shift gears: What's clearer about revised USP Chapter <797>?
Four Ways USP Chapter <797>'s Revision Is Clearer
In its 2008 version, USP <797> was a great reference and standard, but there were elements that sometimes were confusing or misunderstood. The revision has brought clarity to these items, allowing us (and those who inspect us) to be right on target in meeting the chapter intent. Here are the four areas that are clearer:
- CSP CLASSIFICATIONS: The risk classification in old 797 was well-known, but there was some confusion between low and medium risk classifications. For example: Some people interpreted compounding a batch as “low risk,” while others would consider it “medium risk." In the revision, risk classification is replaced by a category system, which is clear cut: Category 1s are prepped in an SCA, and Category 2 items are to be made in a full cleanroom suite.
- CLEANING AND DISINFECTION: Most of us probably won’t have to change much in terms of our cleaning solutions and processes for the PECs, but there was just enough ambiguity in the previous chapter to generate questions both from hospitals and inspectors. Old chapter language suggested we needed to use a germicidal or sporicidal, even just to sanitize the deck throughout the day. (Some surveyors even expected it to be the entire hood and documented each time!) That's changed, and the revision makes things clearer.
Today, we only need to use sterile alcohol to disinfect and sanitize the deck regularly, or every 30 minutes. It's also now clear that regular sanitizing step is just sterile alcohol and does not need to be logged. Finally, the term “germicidal” is no longer used. It’s now a "one-step disinfectant cleaner." - MATERIALS HANDLING: The revision now gives us specific steps for wiping supplies, which is a two-step process when transitioning from a dirty to a cleaner environment. When vials are moved from a dirtier, general pharmacy into the cleaner SEC, they must be wiped with a one-step disinfectant cleaner, a sporicidal one-step, or sterile alcohol. While it’s up to you to define your SOP, remember you must account for your dwell times if you don’t choose alcohol. SEC into the PEC, only sterile alcohol is allowed.
- INCUBATION OF MEDIA: The temperatures and intervals in the old chapter version for incubation left room for interpretation. Now, it’s well-defined, as you can see in the slide below.
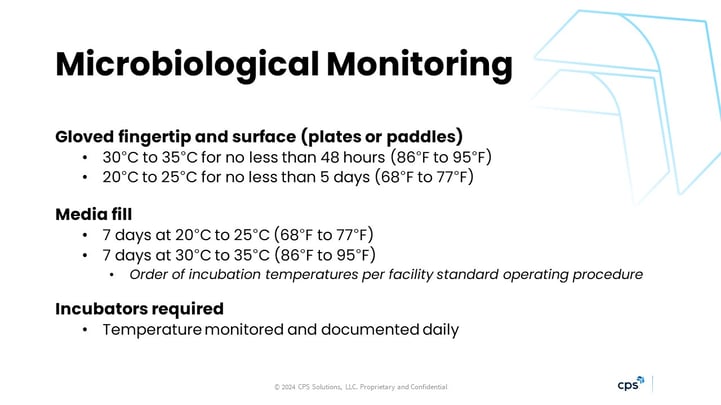 To summarize: Warmer—then cooler—for plates and paddles. Cooler—then warmer—for media fill bags. I believe the dual temperature scheme is meant to tease out both bacterial and fungal growth in one sample.
To summarize: Warmer—then cooler—for plates and paddles. Cooler—then warmer—for media fill bags. I believe the dual temperature scheme is meant to tease out both bacterial and fungal growth in one sample.
Additionally, a late change allowed us to switch the order for media fill bags as defined in SOPs. I still see or get questions about the 20-25 degrees Celsius…this is “room temperature,” but that doesn’t mean we can just store the media on a countertop or in a drawer. The chapter is clear: It still needs to be in a controlled incubator.
Do you have more questions about the revised USP <797>? We have answers.

 All Posts
All Posts