December 5, 2023
The revised USP <797> has been enforceable for just over a month now. Here are more changes to focus on.
By: Chris Beebe
With the revised USP <797> now enforceable, we’ve been focusing on the changes in four categories here at CPS (I call them “buckets”):
- What's clearer?
- What’s more challenging?
- What’s easier?
- What’s new?
In Part 1, I discussed some of the new standards and concepts in the revised (now current!) USP chapter <797>. These are things that weren’t present in the previous version but are now. But wait – there’s even more to cover on stock solutions, HEPAs and air returns, and records and documentation requirements.
Additional New USP <797> Requirements You Need to Know
In part 1, I identified four areas of <797> which hadn't been addressed before, and that stand out to me. Here are three additional areas that you should pay special attention to:
- Stock Solutions
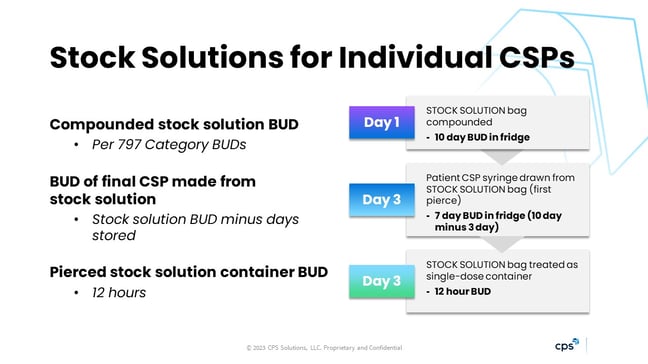
<797> now addresses stock solutions, and I mostly see them used in pediatrics. Think of cefotaxime: when multiple small-volume syringes of this diluted drug are needed, a larger stock bag of the cefotaxime in the same concentration can be made. Then, the individual syringes are drawn out and dispensed.
Revised <797> and the FAQ clearly address the beyond-use dates (BUDs) related to this process, as seen in the image below. The stock solution of the cefotaxime is compounded as a Category 2 CSP, so it gets 10 days in the fridge. On day 3, it is retrieved and the first batch of syringes is made from it. The BUD for these is 7 days, which is what remains on the original stock bag. However, that stock bag is also a single-dose container, which has a 12-hour BUD when pierced in the hood; after that time the stock bag must be discarded.
Facilities that deploy this process will need to look at their workflow to optimize it and minimize any stock solution waste.
- HEPAs and Air Returns
HEPA filtered air has always been required for cleanrooms, and now the filters specifically need to be placed at the ceiling level and not upstream. Having them upstream allowed a length of ductwork where things could grow and blow into the room, so this is definitely a safety improvement.
There is a brand new item related to room air movement, requiring that returns in the rooms must be low on the wall to enhance downward dilution of clean air. That’s great for new construction but what about your rooms that don’t have this? Per the chapter, an air visualization study must be done to see if there’s stagnant air.
But what exactly is ‘low’ and how do you quantify ‘stagnant’? Is it up to tester interpretation? And would this fail certification of an otherwise compliant room? In my conversations with certifiers, it only would need to be done once (not every six months) and it would point out opportunities for improvement, but not fail the room into SCA status. Be sure to inquire about this with your certifier and see how they plan to address it.
-
Records and Documentation
A new addition to <797> is the specific requirement for records and documentation of compounded sterile products. This has been in <795> for non-sterile compounding and is now for sterile too. There are two types of records you’ll need to produce. The Master Formulation Record is the general recipe and is really only needed for items that you batch. The intent is to describe the step-by-step process for the compounder to follow.
For every compound including patient-specific and one-offs, a Compounding Record is required. This is specific to the actual preparation and includes details like the lots and expirations of each component and the identity of the compounder and checker. Some states already require this, but it now will be needed everywhere.
This information can be captured manually or in an electronic system; I do recommend this be done electronically if you can, as there will be a lot of paper otherwise.
With so many new requirements and uncertainties, I recommend you find a trusted partner to help you through this next phase of compliance preparation.
I know we at the CPS team would welcome the opportunity to help.

 All Posts
All Posts